Regenerative Medicine
The field of regenerative medicine is growing rapidly. Regenerative medicine utilizes the body’s natural healing abilities to treat diseases and improve the quality of life. It is estimated that up to 128 million individuals might benefit from regenerative medicine therapy, or almost 1 in 3 individuals, in the US alone1.
The need to relieve suffering and reduce healthcare costs is an enormous motivator to rapidly bring stem cell therapies to the clinic offices throughout the U.S. Cellular therapies are among the most studied products of potential use in regenerative medicine research. Clinical studies involving cells focus on deploying them to assist in the repair and recovery from disease/disorders. Certain cells, such as immune, chondrocyte, islet, and stem cells, have been shown to release proteins or other factors that help activate endogenous repair.
Cord Blood
Umbilical cord blood is a rich source of many cell types including immune and progenitor cells, endothelial cells, and 2 different types of stem cells. It is also a rich source of proteins and growth factors designed to sustain life. Umbilical cord blood is highly oxygenated and rich in nutrients and is transferred from the placenta to the fetus for 9 months.
Transplanting stem cells from umbilical cord blood to address a variety of hematology and oncology disorders has been used successfully in improving many lives, with over 30,000 transplants worldwide2. Cord blood is already used to treat over 80 diseases and researchers are exploring other potential uses3.
Newer potential therapeutic treatments with cord blood focus on repair of other damaged tissues and organs in the body, including in treatments for conditions such as brain injury and juvenile diabetes. Other areas being studied include multiple sclerosis, rheumatoid arthritis, cardiovascular conditions, sickle cell disease, corneal regeneration, cartilage and tendon repair, cerebral palsy, plus many more3.
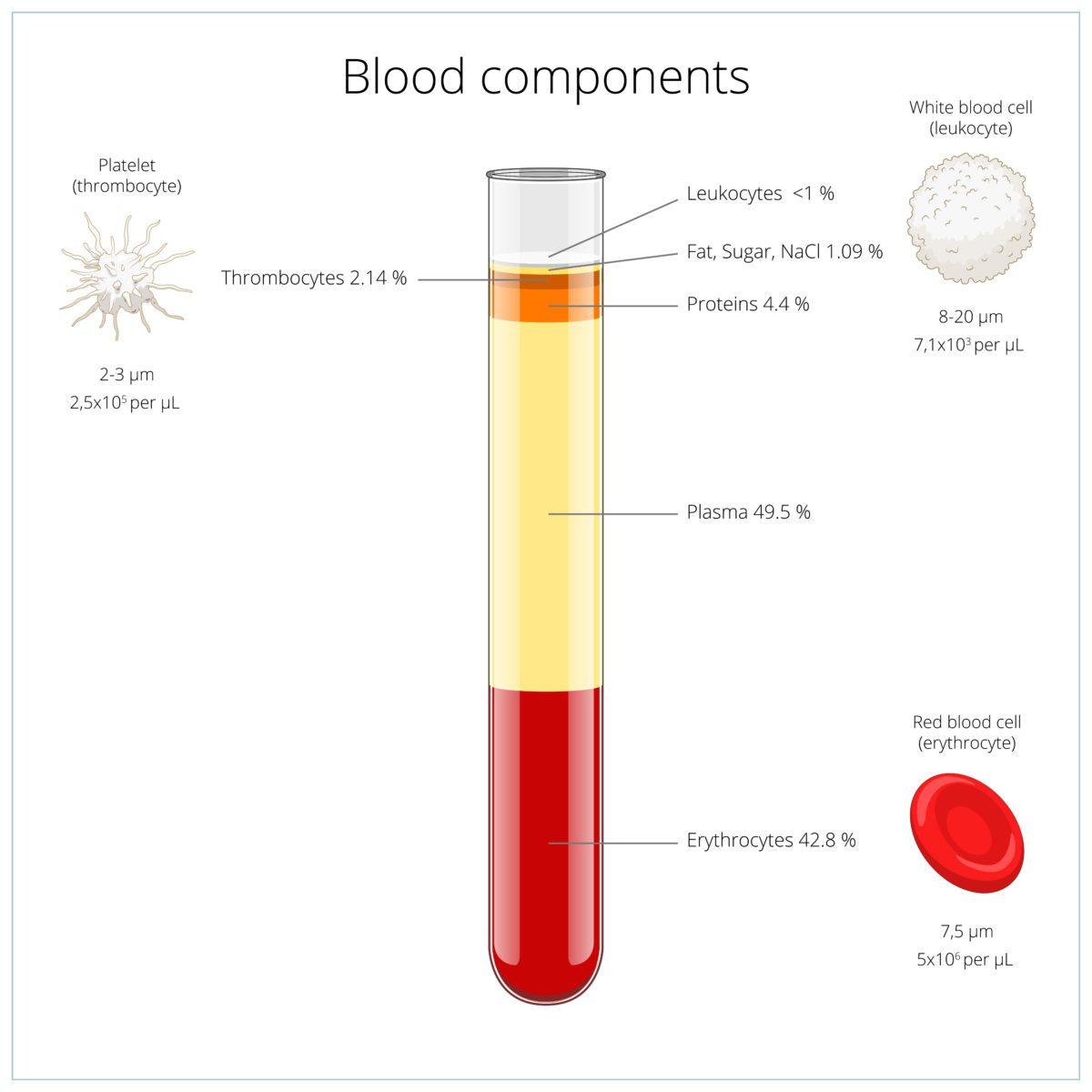
Cord for Life®and Clinical Research
Cord for Life®, is a full-service cord blood bank with over 25 years of experience. We have released over 2,200 cord blood units for transplants, research, and clinical trials. We are now helping to accelerate research into regenerative medicine by sponsoring clinical trials for new applications.
We have received IND approval by the FDA to conduct a dose ranging study to assess the safety, tolerability, preliminary efficacy, and dose effect of its allogeneic individual umbilical cord blood-derived stem cell product in patients with lower back pain of the Sacroiliac Joint (SIJ). The initial trial includes nine patients with lower back pain, who will receive a range of dosing regimens using our PREMIERMAXCB ® product. Following injection, patients will be monitored for 12 months.

About PREMIERMAXCB ®
PREMIERMAXCB ® is a newly developed investigational biological therapeutic derived from umbilical cord blood. The PREMIERMAXCB ® product line is ideal for smaller, localized treatments. Each vial of PREMIERMAXCB ® contains 1mL/1cc of a cellular and protein cocktail that has demonstrated promising results in early research testing.
PREMIERMAXCB ® comes in 3 doses:
- Silver (an average of 8 Million total nucleated cells)
- Gold (an average of 15 Million total nucleated cells), and
- Platinum (an average of 30 Million total nucleated cells).
Manufactured under cGMP conditions in our state-of-the-art clean room facility, PREMIERMAXCB® is tested to the highest standards for safety, quality, identity, purity, and potency.
PREMIERMAXCB® is currently available for research use only.
Our Product Research and Development Team
Our Product Research and Development Team is comprised of individuals who participated in helping us achieve our IND by providing valuable input, product testing, and guidance.
Diego Anguiano, MS
Lab Operations Manager / Researcher Associate of Turn.bio a Stem cell and regenerative medicine scientist. Senior cord blood stem cell advisor for multiple companies. Eight years of cellular biology experience with an emphasis in global business development.
Asawari Bapat, MD
Asawari (Asa) has 20+ yrs of “Leadership” experience globally, in executing projects, operating facilities; Diagnostic labs, facilities for Cord Blood Banks, Blood and Plasma Products, Transfusion medicine, Transplants, Biotherapies and Regenerative Medicine.
She has been spearheading Labs, Cord Blood Banks, Cell and Gene Therapy Facilities, and Biotech Companies towards quality, compliance, and financial success. Asa performs a critical part in creating, innovating, designing and establishing novel technologies for Clinical Trials in Regenerative Medicine, Biotherapies and Medical Devices as a subject matter expert, Consultant, Medical Director, for Pre-IND’s, IND’s, BLA’s, IRB’s, ATMP’s and is instrumental in document submissions and representation of clients at FDA, CBER, CDER, TRIP submissions, INTERACT and Type A,B and C meetings for Expedited pathways such as Fast Track Approvals, RMAT’s.
Personal Background:
- Asa is a Medical Doctor (MBBS, Bachelor of Medicine and Bachelor of Surgery, India), a Clinical Pathologist (specialization in Transfusions, Transplants, Lab Medicine, Immunology, CPS, India).
- Asa received training in Cell and Gene Therapies, Biotherapies in Sydney Australia, India and in the US.
- She is an international ambassador of AABB ( Association for the Advancement of Blood and Biotherapies) and a committee member (Cell Therapy Subsection Coordinating Committee) representing the global experts for Biotherapies.
- Speaker, Moderator, Panelists, Committee member at international conferences. Author of books and peer reviewed international publications in Journals of significance
Scott Faulkner, MD
Dr. Faulkner, MD, is an Internal Medicine specialist in Larkspur, Colorado. He graduated with honors in 1995. Having more than 25 years of diverse experiences, especially in INTERNAL MEDICINE, Dr. Faulkner affiliates with many hospitals including Delta County Memorial Hospital, Castle Rock Adventist Hospital, Grand River Hospital District, cooperates with many other doctors and specialists in medical group Portercare Adventist Health System and Centura Health-St Thomas More Hospital.
Kevin Kong, PharmD
Dr. Kong is an Oncology Pharmacist Specialist with the Chao Family NCI Comprehensive Cancer Center University of California. He received his PharmD from the University of the Pacific. His clinical residency was at the University of California, San Diego.
His expertise is in Clinical Development, Regulatory, Operations & Manufacturing. His industry background is in Healthcare, Biotechnology and Cosmetics. He is a partner/investor at LMK Healthcare, LLC.
Keith March, MD, PhD, FACC
Keith March, MD, PhD, FACC a cardiologist and national leader in regenerative medicine was recruited by the University of Florida, Gainesville, from the Indiana University School of Medicine to launch a Center for Regenerative Medicine to develop lifesaving therapies “to heal the body from within” using stem cells that repair damaged tissue and organs.
Dr. March’s research has led to about 55 worldwide patents, including more than 20 in the United States. He invented the Closer, a device that has been used in more than 8 million heart catheterizations to close an arterial puncture wound created by the procedure. He has focused his research on vascular biology, particularly in the study of adipose, or fat-derived, stem cells. March’s research has led to more than 150 manuscript publications.
March also has worked on the key insight that stem cells’ natural role is to repair vascular damage, allowing blood vessels to grow and enhance the blood supply.
Rajen Naidoo, MD
Dr. Naidoo brings a 30-year history in the biosciences to his role as Chief Medical Officer of New Life Regenerative Medicine and World Biotech Hong Kong. Dr. Naidoo has served as an executive of several biotech companies for the past eight years and is also the Principal and Partner of Apollo Regenerative Solutions, a scientific consulting company.
A physician-scientist with a doctorate in Medicine from Yale University, Dr. Naidoo also holds biomedical engineering degrees and completed Orthopedic Surgical training at Albert Einstein College of Medicine.
Dr. Naidoo brings to our company the unique blend of long-term scientific research and clinical experience with stem cell and regenerative medicine and possesses medical licenses in multiple US states and countries and carries a vast demographic of clinical use of regenerative products.
Dr. Naidoo is a native of South Africa and in his early years proudly served the revolutionary army of Nelson Mandela in South Africa’s struggle for freedom.
Milad Riazifar, Phd
Dr. Riazifar has worked on stem cells over a decade and on exosomes a subclass of stem cell derived extracellular vesicles since 2012. During his PhD studies at the Stem Cell Research Center at the University of California Irvine, he pioneered a cell-free exosome-based approach to treat Multiple Sclerosis. At City of Hope, he was part of a clinical translation team initiating the world’s first clinical trial using stem cell exosomes for the treatment of type I diabetes.
Dr. Riazifar has extensive experience in manufacturing and characterizing pharmaceutical-grade exosomes, and has developed numerous in vitro potency assays, and is overseeing IND-enabling preclinical studies. He has published numerous high-impact articles on stem cells and stem cell-derived exosomes and has consulted for regenerative medicine companies. Dr. Riazifar received his Ph.D in pharmaceutical sciences from UC Irvine.
Partnership Opportunities
Our business philosophy is simple, “Collaborate and Partner.” We are interested in synergistic partnerships that enable both parties to share resources, expertise, and assets for the purpose of delivering an exceptional standard of service to their clients or patients.
Since 1995, Cord for Life® has been a leader in cord blood collection, processing and storage. We were the first cord blood bank to accept both private storage and publicly donated cord blood collections from anywhere in the continental United States. Our team of scientists and cord blood banking professionals are leaders in the cord blood collection community who have helped clients around the world successfully launch and grow their organization’s cord blood collections. Our team members are passionate about our mission to Save Lives.
For more information about clinical research:
For more information about investment opportunities:
Citations
- Harris, D. T. 2014. Stem Cell Banking for Regenerative and Personalized Medicine. Biomedicines. 2014 Mar; 2(1): 50–79
- Hill GR, Betts BC, Tkachev V, Kean LS, Blazar BR. Current Concepts and Advances in Graft-Versus-Host Disease Immunology. Annu Rev Immunol. 2021 Apr 26;39:19-49. doi: 10.1146/annurev-immunol-102119-073227. Epub 2021 Jan 11. PMID: 33428454; PMCID: PMC8085043.
- Parents Guide to Cord Blood https://parentsguidecordblood.org/en/diseases, F. Verter, PhD, 2014

Our purpose is to provide the expectant mothers with information necessary to discover the lifesaving potential of umbilical cord blood.
Cord for Life®
Resources
Copyright Cord for Life 2019. All rights reserved.
